Ascendco + Prisma Health Collaborative Partnership
The downstream effects of compromised packaging can hamper operational efficiencies in surgical services departments. They can cause delays or cancellations of procedures, incur additional reprocessing costs and burdens to Sterile Processing teams, and erode trust between Sterile Processing and the Operating Room. In addition, repeated events may lead Sterile Processing teams to increase available stock on hand, through purchase, consignment, or loaners, to compensate for defective products. Through the help of analytics, a large facility in the Southeast utilized data to study trends, identify where this issue was occurring and targeted those areas to implement process improvements.
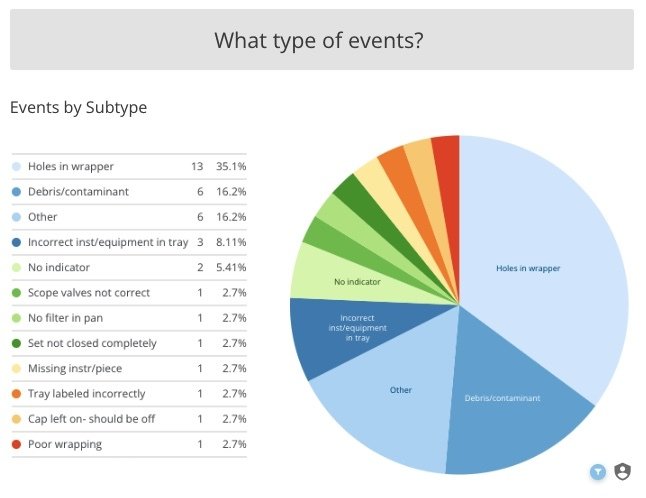
Critical medical devices must be terminally sterilized and packaged in barrier systems designed to maintain sterility, like rigid sterilization containers, polypropylene wrap, or paper/plastic pouches. After an item is sterilized, it can be handled multiple times before use. Each time the item is handled, the packaging can be punctured or torn – this is most common with wrapped items. This compromises the sterility of the item and it must be fully reprocessed. Therefore a common initiative to decrease quality events in the OR is to target improvement initiates to eliminate holes in wraps.
This case study focuses on the improvement work at Prisma Health Greenville Memorial Hospital in Greenville, South Carolina. Sherry Gravely, the Nurse Manager of Sterile Processing, utilized a data-driven approach to identify and solve problems. After documenting quality event data for a year, the Prisma Health Greenville team used the insights provided by the Ascendco Analytics platform to identify the most common quality event — holes in wraps. Developing solutions to this problem became the Sterile Processing team’s priority.
“Holes in Wrapper” Events
Events at Prisma Greenville Memorial Hospital, by type, prior to process improvement project. “Holes in Wrapper” accounted for 35.1% of events.
The next step was identifying which wrapped sets were causing the most issues. Factors such as weight, storage location, design of the inner container, or how frequently the set is handled before use make some wrapped sets more prone to packaging defects. The Prisma Health Greenville team used the Ascendco platform to develop a “Top Five” list of wrapped sets found with compromised packaging. Because rigid sterilization containers offer greater protection during storage and handling, the team decided to convert the barrier system of their “Top 5” sets from wraps to rigid containers.
In order to complete this conversion, containers needed to be purchased, which is a major investment for a facility. A single container can cost hundreds of dollars, depending on the size, number of inner baskets, accessories, etc. The set that had the highest recorded number of holes in wrappers, Laparoscopic Instruments, has 45 inventory instances. Meaning 45 containers needed to be purchased to perform the conversion. In total, the cost of the containers purchased for this project was nearly $70,000. In order to avoid a capital purchase or a large, one-time hit to the books, the team purchased 3-4 containers each month over the course of a year. This also allowed the team to convert the containers without impacting operations.
Reduction of quality event rates
from January, 2020 through December, 2021.
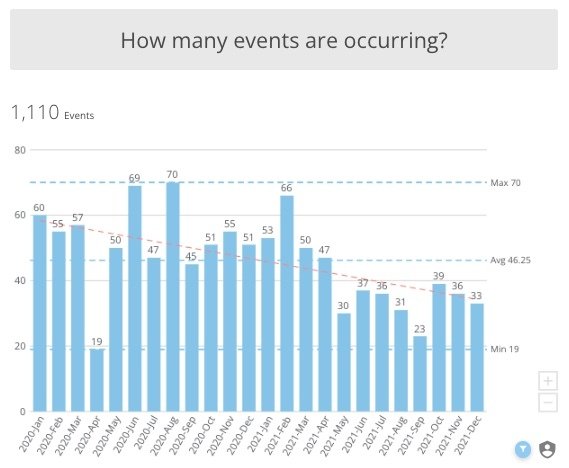
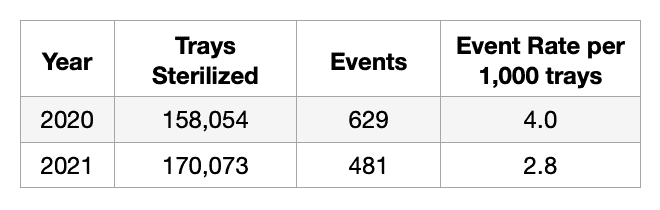
Because the success of this project relied on a substantial container purchase, it was of utmost importance to have supporting data. The detailed event information captured by the Prisma Health Greenville team combined with the analytical tools from Ascendco provided clear, concise, and actionable information. Sherry utilized this information during conversations with department leadership to justify the resources to make the purchase.
At the completion of this project, the Prisma Health Greenville Sterile Processing Department saw a reduction in the number of holes in wrappers and quality events overall. This reduction is not only reflected in the total number of events, but also in the event rate per sterilized tray. In addition, the team realized the soft benefits of a significantly improved relationship between Sterile Processing and the Operating Room. The Sterile Processing team became more confident that they could provide a defect-free product and the Operating Room began to trust the Sterile Processing team’s work. Clearly defined goals, strong leadership, and actionable information made this project a successful one and provided a blueprint for other improvement opportunities. Together they have begun targeting their next improvement initiative – missing instruments.
