BY JOAN M. SPEAR, MBA, RN, CNOR, CRCST, INDEPENDENT CONSULTANT
A facility’s count sheets (also known as recipes or tray sheets) can provide an accurate list of contents communicated in a clear, concise and descriptive manner. This is particularly true when the facility adopts standardized instrument nomenclature and formatting. Using a standard instrument- and set-naming convention that provides names everyone can recognize—along with instrument size, length, type of jaws and other identifying information—is an effective way to take control of instrument count sheets and aid communication between team members in Sterile Processing (SP) and the Operating Room (OR).
Instrument Names and Formatting
When creating a count sheet, instrument names and descriptions and a standard formatting are critical to help staff reliably produce an instrument set with the correct instruments. A count sheet can be used by the SP team as a checklist, much like a surgical checklist. The World Health Organization (WHO) introduced the WHO Surgical Safety Checklist in 2009 to provide guidance for healthcare organizations for reducing complications in surgery.1 While barriers existed in the U.S. (2) the concept of a surgical checklist was supported by many organizations, including the American Academy of Orthopaedic Surgeons (AAOS) (3) the Association of periOperative Registered Nurses (AORN), and the Joint Commission (TJC), and templates were widely distributed and implemented. SP personnel use count sheets to ensure the correct instruments are
in specific sets, and the count sheet provides communication between SP and the OR team regarding contents of a specific instrument set. Reliability is important for the surgical team, and missing instruments can result in a procedural delay while the surgical team waits for a replacement.4 Patients depend on the surgical team’s readiness for their procedures, and the surgical team depends on the SP team to provide complete and correct instrument sets. Using catalog names can help reduce confusion and is a solution used by facility supply chain professionals and some clinical personnel.
Conflicts in instrument nomenclature exist between instrument manufacturers as well. Mosquito forceps are just one example. One manufacturer lists the instrument as Halstead mosquito forceps 4 5/8” (5), while another lists the same instrument as Halstead micro- line artery forceps 5”.6 Although there is a 1/8” difference in length, these two devices would be considered matching instruments.
Instrument nicknames may also cause confusion, such as when one OR team member knows a surgeon’s nickname for an instrument and other team members do not. This can result in OR team members calling SP for that instrument, using the surgeon’s special nickname. Stress and confusion can arise in the absence of standardized instrument nomenclature. Some common examples of instrument nicknames are shown in Figure 1.
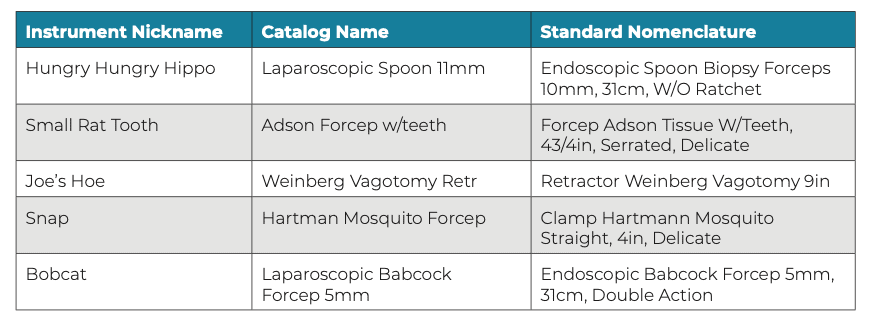
Figure 1: Examples of instrument nicknames, which can cause confusion
Examples of well-constructed instrument names are shown in Figures 2 and 3. Note how the type of instrument, classification, specific characteristics (i.e., curved or straight) and size are all included in a standard format with standardized language.

Figure 2: Rongeur, Kerrison, 1mm, 40° up, 7″

Figure 3: Scissor, Mayo, str, B_B, 63⁄4”, TC
A standardized format could be the order in which the instrument name and details appear or another agreed- upon system. Formats and names vary from one instrument manufacturer to another, resulting in challenges when cross-referencing, ordering and updating count sheets. Names of surgical instruments may come from the surgeon who designed the instrument, making it more difficult to put that name in a standard format.7 Emily Tchiblakian, Senior Director of Surgical Services at Community Health Systems, recognized that instrument name standardization was necessary for the reorder process, building preference lists in an electronic system, accomplishing set optimization, and having accurate count sheets. She addressed the issue by forming a focus group to determine an instrument naming convention that all facilities and departments in their healthcare system would use. Staff at newly formed health systems, as well as those that have integrated with another hospital or surgery center, are implementing or changing an instrument management system, or are sharing count sheets with colleague in other organizations. This may lead staff to encounter challenges in regard to instrument name recognition. Potential barriers can include resistance or opposition from those who prefer to use favorite nicknames as opposed to official instrument names. A simple solution can be adding an “also known as” (aka) column or including the nickname in parentheses at the end of the standard instrument name. Instrument management systems can support standardization of instrument nomenclature and count sheet formatting, making set assembly in the SPD and set use in the OR more predictable and reliable. Each instrument management system may also have a unique solution for overcoming the fear of losing instrument nicknames.
Avoiding communication issues caused by using multiple names for the same instrument should be the goal of the OR and SP teams as well as supply chain professionals and medical device manufacturers. When each instrument has the same name across all services and facilities within a health system, improved efficiencies will result.
One study was undertaken at a hospital where 20 instrument-related quality events occurred each month, resulting in surgical delays.8 Quality events included incorrect instruments, missing instruments and the wrong number of instruments. This study highlighted the need for using correct count sheets as a checklist for all personnel to access. Both SP and OR personnel must understand the count sheet language and be able to correctly identify each instrument using its name and description. Count sheet descriptions must also be specific. For example, using the term “curette” as the description does not provide enough information to identify the correct instrument. A micro curette is very different from a spinal curette. Figure 4 provides an example of a count sheet with standard instrument nomenclature and formatting.
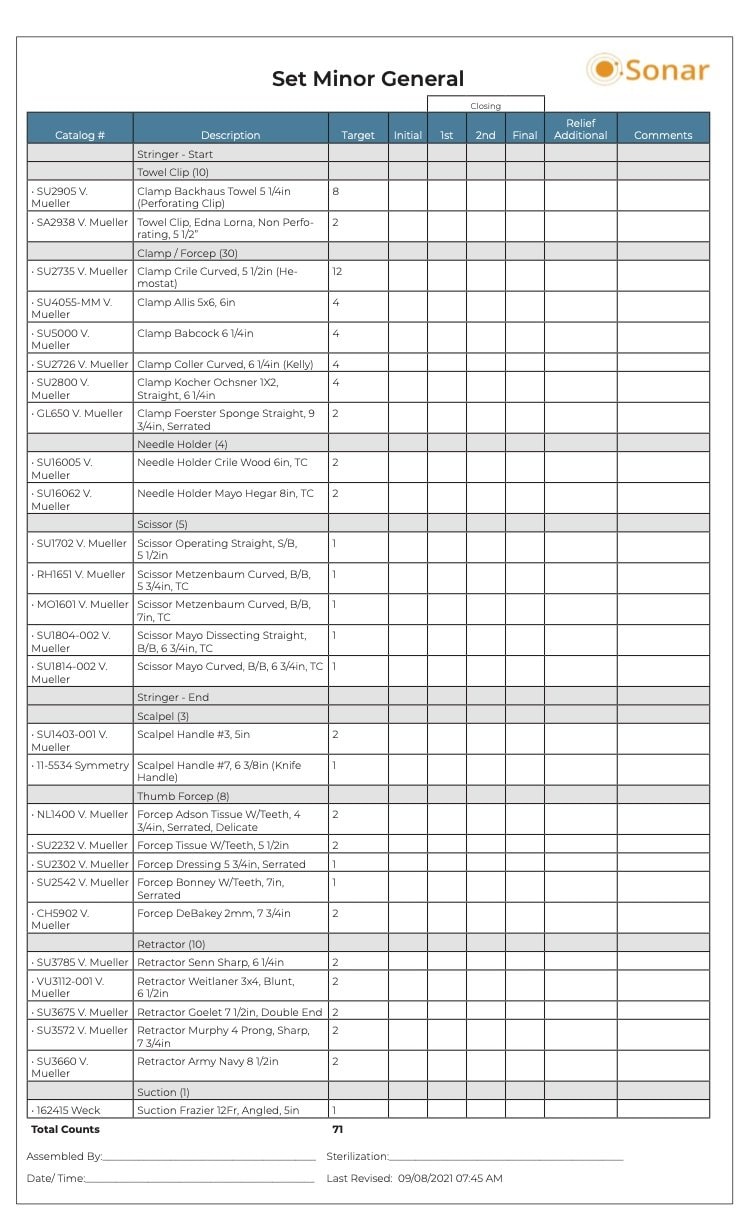
Figure 4: Example of count sheet with standard instrument nomenclature
Conclusion
Communication around surgical instruments and instrument sets is important. The surgical team drives the contents of the sets, and both SP and OR professionals need to collaborate to standardize instrument nomenclature and count sheet formatting to reduce confusion and increase accuracy. All stakeholders are important in the process.
The word communication was derived from the Latin word communis, which means “common.”9 To quote author and communication studies expert Wilbur Schramm, “We are trying to establish a ‘commonness’ with someone.” SP professionals can take the lead on establishing a commonness with the OR team by standardizing surgical instrument names and count sheet formats.
This article was featured in the January/February 2022 edition of HSPA’s PROCESS Magazine.
References
- World Health Organization. “WHO Surgical Safety Checklist,” https://www.who.int/ teams/integrated-health-services/patient- safety/research/safe-surgery/tool-and- resources.
- Jain, D., Sharma, R., and Reddy, S. “WHO Safe Surgery Checklist: Barriers to Universal Acceptance,” Journal of Anesthesiology Clinical Pharmacology 34, no. 1 (Jan–March 2018): 7–10, https://www.ncbi.nlm.nih.gov/ pmc/articles/PMC5885453/.
- Panesar, S. et al. “Can the Surgical Checklist Reduce the Risk of Wrong Site Surgery in Orthopaedics? Can the Checklist Help? Supporting Evidence from Analysis ofa National Patient Incident Reporting System,” Journal of Orthopaedic Surgery and Research 6, 18 (2011), https://doi. org/10.1186/1749-799X-6-18.
- Spear, J. “Instrument Count Sheets and Set Reviews as Patient Safety Tools,” AORN Journal 104, no. 6 (December 2016): 588– 592, https://doi.org/10.1016/j.aorn.2016.10.007.
- Integra. “Halstead Mosquito Forcep,” https://www.integralife.com/ halstead-mosquito-forcep/product/ surgical-instruments-hospitals- surgery-centers-tissue-banks-integra-ent- general-forceps-halstead-mosquito-forcep.
- Becton, Dickinson and Company (BD). “Halsted Micro-Line Artery Forceps,” https://www.bd.com/en-us/offerings/ capabilities/surgical-instruments/open- instrumentation/halsted-micro-line-artery- forceps/su2698?return=true.
- Gagnieur, P. et al. “De l’histoire des chirurgiens cachés derrière nos instruments du quotidien. Partie 4:
la rhinoplastie.” [French] Annales de Chirurgie Plastique Esthétique 65, no. 4 (2020): 271–276, https://doi.org/10.1016/j. anplas.2020.05.005. - Lallatin, J. and Sharke, T. “Count Sheets in Instrument Trays Help Decrease Surgical Delays.” AORN Journal (Special Focus Issue: AORN Pre-Conference) 111, no. 1 (January 2020): P11–P13, https://doi.org/10.1002/ aorn.12937.
- Madhuri, P. “The word communication,” Academia, https://www.academia. edu/34838370/.
